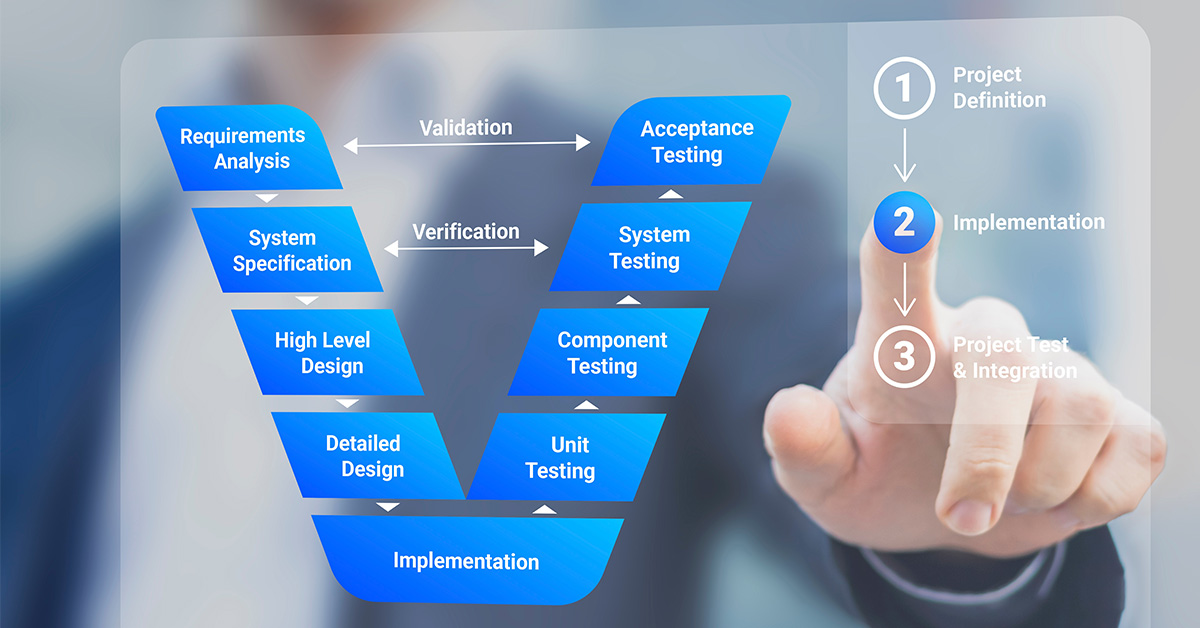
The medical device manufacturing landscape is characterized by its rigorous regulatory environment, designed to ensure the highest levels of product effectiveness and patient safety. Central to successfully navigating this environment are Design Verification and Validation (V&V), which serve as critical pillars in both product development and regulatory compliance.
Design V&V are systematic approaches utilized in the medical device industry to ensure that a product meets the needs of the end-user and complies with all regulatory requirements. Verification confirms that the design outputs meet the input requirements, while Validation ensures that the device conforms to defined intended uses. Both processes are integral to the product lifecycle — fostering innovation, ensuring safety, and maintaining compliance.
The global medical device market is governed by various regulatory bodies, each with its demands for ensuring product safety and efficacy. The U.S. Food and Drug Administration (FDA) and the European Union's Medical Device Regulation (EU MDR) are two such entities setting rigorous standards for medical devices. Adherence to these standards through effective V&V not only facilitates market access but also significantly reduces the risk of post-market issues, reinforcing patient safety and product reliability.
Understanding Regulatory Requirements (FDA, EU MDR, etc.)
To ensure compliance with internationally recognized standards, such as the FDA 21 CFR 820.30, which focuses on Design Control, and ISO 13485's Clause 7.3 on Design and Development, it is imperative for manufacturers to gain a thorough understanding of the distinct yet overlapping requirements outlined by these regulatory frameworks. Both sets of regulations place a strong emphasis on the need for comprehensive documentation that must be meticulously maintained throughout the entire design and development process. However, the primary differences between the two stem from their geographical applicability and the specifics of their execution, which may vary depending on the regulatory environment. Understanding these nuances is crucial for manufacturers to navigate the complex landscape of global compliance, thereby ensuring that their products meet the highest standards of safety and efficacy.
Risk Management in Verification and Validation
Risk management plays a crucial role in V&V processes, mandating that manufacturers proactively identify and mitigate potential risks related to their medical devices. By adopting a risk-based approach from the initial stages of development, manufacturers can not only guarantee compliance with strict regulatory standards but also prioritize patient safety at every stage of the product's lifecycle. This involves a thorough assessment of potential hazards and their impacts, ensuring that every aspect of the product's design and functionality is scrutinized for safety before reaching the market.
Integrating Human Factors Engineering
By incorporating Human Factors Engineering (HFE) principles into the V&V processes, developers can ensure that the medical devices will perform as intended when operated by the end user. This integration not only enhances device safety by reducing the likelihood of user error but also improves the overall usability, making the devices more intuitive and user-friendly. Consequently, application of HFE principles in the development phase contributes significantly to the successful deployment of medical devices, ultimately leading to better patient outcomes and satisfaction.
The landscape of medical device manufacturing is continually evolving, driven by technological advancements and changing regulatory requirements.
Effective Design Verification and Validation are foundational to the success of medical device manufacturers in the global market. By adhering to regulatory requirements, employing risk management strategies, and integrating human factors engineering, companies can ensure their products are effective, safe, and compliant. With the medical device industry poised for more innovations and regulatory evolutions, staying abreast of best practices in V&V will remain critical for manufacturers worldwide.
Are you ready to ensure your medical device design complies with key regulations? Start your path to compliance today. Unlock your product's success with our help—begin your journey now.